Abstract
Philadelphia-negative myeloproliferative neoplasms (MPN) are closely related stem cell disorders. Transitions between disease entities are common, shaping a "biological continuum" from an early stage with a relative milder phenotype (polycythemia vera, PV and essential thrombocythemia, ET) toward an advanced phase, termed secondary myelofibrosis (sMF). Currently, no clinical or laboratory parameter can predict if, and over what period, a PV or ET will progress toward sMF.
Chronic inflammation plays a pivotal role in MPN pathogenesis, triggering neoplastic transformation and driving clonal evolution to end-stage disease (Hasselbalch C, Blood 2012) . Tefferi et al. demonstrated that myelofibrosis (MF) patients display higher levels of pro-inflammatory cytokines than healthy subjects, with a relevant role of IL-8 and sIL-2R in phenotypic correlations and disease prognosis (Tefferi et al. JCO 2011) and - for MCP-1, sIL-2R, IL-15 and IL-8 - in predicting response to pomalidomide (Pardanani et al. Am. J. Hematol 2011).
We focused on MCP-1 since it is the main chemotactic factor for monocyte migration in sites of inflammation, contributing to organ fibrotic changes (Deshmane et al. J Interferon Cytokine Res. 2009) . An A to G single nucleotide polymorphism (SNP) in MCP-1 enhancer region at position -2518 (rs1024611) was found to be associated with higher serum MCP-1 levels and increased susceptibility to chronic infectious disease, atherosclerosis, autoimmune disorders and other chronic inflammatory conditions (Colobran R. et al. Clin Exp Immunol. 2007) .
In this study, we evaluated genotypic and allelic frequencies of the -2518 A/G SNP of MCP-1 in 159 Caucasian MPN patients, of which 38 PV, 58 ET and 63 MF [41 primary (PMF) and 22 secondary (sMF)] and 149 Caucasian age- and sex-matched healthy subjects (CTRL). Genomic DNA was extracted from 200 μl of whole blood and the SNP detected by TaqMan® SNP Genotyping Assay. Chi-Square test was used for statistical comparisons.
Median age was 72 yrs for PV (range 35-75), 59 yrs for ET (range 17-86), 74 yrs for MF (range 29-88) and 63 yrs for CTRL (range 29-85); 60% of PV were male, vs 49% of ET, 59% of MF and 56% of CTRL.
Genotypic frequencies of CTRL followed the Hardy-Weinberg equilibrium and were similar to those reported in the literature (Szalai et al. Atherosclerosis 2001, Muhlbauer et al. Gastroenterology 2003). We did not find statistical differences in both genotypic and allelic frequencies among PV, ET, MF, total MPN patients and CTRL (Tab. 1 and 2). Of note, only one out of 58 ET (2%) was homozygous for the polymorphic allele, consistently with the milder phenotype of this disease as compared to PV and MF.
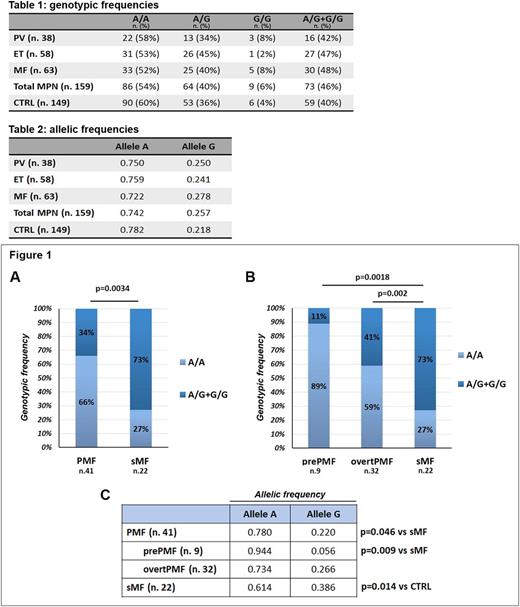
Focusing on MF, we found that the frequency of polymorphic genotypes was significantly higher in sMF vs PMF (16/22, 73% vs 14/41, 34%, p=0.0034, Fig.1A). This statistical difference was also confirmed when splitting PMF population into prePMF and overtPMF, with an increased frequency of allele G-carriers in sMF as compared to both prePMF (1/9, 11%, p=0.0018) and overtPMF (13/32, 41%, p=0.02, Fig. 1B).
Statistical differences in allele A and G frequencies were observed for PMF vs sMF (p=0.046,) and for prePMF vs sMF (p=0.009) (Fig.1C). Of note, both genotypic and allelic frequencies of sMF patients were statistically different from CTRL (p=0.011 and p=0.014, respectively).
In addition, in the overall MF population, we described a statistical trend toward association between the SNP and a higher IPSS/DIPSS risk category (A/G+G/G=17/29, 59% in int-2/high risk patients vs 12/33, 36% in low/int-1, p=0.07). Allele G carriers were 15/30 (50%) in bone marrow fibrosis grading ≥ II and 8/23 (35%) in grading 0-I. In sMF, patients harboring the G allele had a median time to progression from the previous PV or ET of 8 yrs (range 1-35) vs 13 yrs (range 8-15) of wild type patients. No association was detected between the SNP and: (i) JAK2V617F mutational status; (ii) history of major thrombotic events.
The described association between -2518 A/G SNP of MCP-1 with sMF is consistent with the concept of sMF as burnout phase of a long process that starts with ET or PV and sees a progressive increase of both local (bone marrow) and systemic inflammation. We can speculate that this SNP may configure as a genetic biomarker that predicts the development of post-PV/ET MF, allowing adequate risk stratification and follow-up.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal